Audit Trail: Ensuring Traceability and Compliance
In many industries, particularly in larger organizations, maintaining an immutable audit trail of all database modifications is not just a best practice, but a legal requirement. Regulations such as US FDA Title 21 CFR Part 11 mandate strict record-keeping and audit trails for companies in the medical and pharmaceutical sectors. However, even without such legal obligations, many companies choose to implement audit trails for enhanced traceability, accountability, and data integrity.
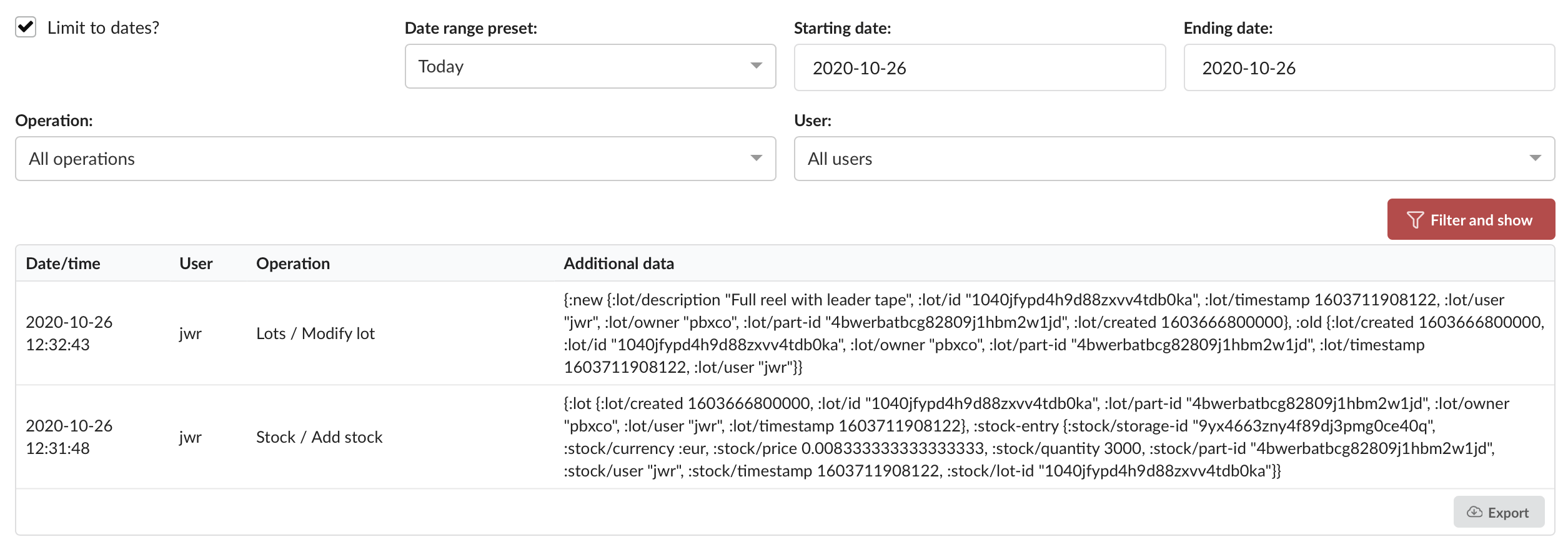
PartsBox's Immutable Audit Trail
PartsBox offers a robust, immutable audit trail feature that automatically records a detailed history of every change made to the database. Each audit trail record includes:
- Timestamp: The exact date and time when the modification occurred.
- User: The user account that performed the operation, allowing for individual accountability.
- Operation: The specific action performed, such as creating, updating, or deleting a record.
- Record Type: The type of record affected, such as a part, storage location, project, or order.
- Record ID: The unique identifier of the affected record.
- Changes: Detailed information about what exactly was changed, including old and new values.
This comprehensive audit trail ensures that no modification goes unnoticed and that there is always a clear, step-by-step history of how the database has evolved over time.
Export and Signature for Regulatory Compliance
To facilitate regulatory compliance and streamline audits, PartsBox allows audit trail data to be easily exported in a standardized format. This exported data can then be digitally signed using secure cryptographic methods, providing an additional layer of integrity and authenticity.
The combination of an immutable audit trail and signed exports makes PartsBox well-suited for use in regulated industries. It provides the necessary tools to meet stringent record-keeping requirements and demonstrate compliance to auditors and regulatory bodies.
Availability and Pricing
The audit trail feature is included in PartsBox's Medical/Pharma plan, which is tailored to the needs of companies in the medical and pharmaceutical industries. For organizations in other sectors that require audit trail functionality, it is available upon request. Please contact our sales team to discuss your specific requirements and pricing.
With PartsBox's audit trail, you can have confidence in the integrity and traceability of your parts inventory data. Whether for regulatory compliance or simply for better data governance, this feature provides an essential tool for managing electronic parts in today's complex and demanding business environment.